Your patients deserve next-generation pregnancy care.
Predict who’s at risk months before symptoms. Individualize their care. Get ahead of preeclampsia.
Encompass™ combines a breakthrough blood test to predict your patient’s personal preeclampsia risk with a preventive action plan and a responsive virtual assistant to help you give them individualized support for the healthiest pregnancy possible.
Breakthrough RNA-science
Powered by the Mirvie RNA Platform, Encompass analyzes thousands of biological signals from the mom, the baby, and the placenta to deliver unmatched predictive insight.
Preventive action plan
Test results are paired with an evidence-based action plan to help reduce their risk. Review it with them to determine the best plan for their pregnancy.
Risk tracking & alerts
Your patients are supported and on track with expert-reviewed guides, medication check-ins, blood pressure monitoring, and alerts when their numbers indicate they need to contact your clinic.
How your patients can order
Ordering the Encompass test before the 18-22 week window is a simple online and at-home process.
Encompass
test kit
blood draw
online
Clinical validation study
A paradigm-shifting study published in Nature Communications in April 2025 shows Encompass can identify 91% of pregnancies that will develop preterm preeclampsia months before symptoms.
Researchers used data from more than 9,000 pregnancies within the multi-center Mirvie-sponsored Miracle of Life prospective study to discover and validate RNA signatures capable of distinguishing between severe and mild hypertensive disorders of pregnancy, including preeclampsia, months before symptoms occur. The study validated the test in women aged 35+ without pre-existing high-risk conditions.
Findings
- Validated RNA signatures that distinguish between severe and mild hypertensive disorders of pregnancy, including preeclampsia, months before clinical presentation.
- Strongly supports the hypothesis that preeclampsia is a condition with multiple underlying causes that lead to a common set of symptoms.
- Demonstrates there is no molecular difference between preeclampsia in late pregnancy and gestational hypertension.
- Risk stratification based on individualized RNA signatures offers a 15x improvement compared to using only maternal clinical and demographic characteristics.
- Validation results indicate that a simple blood test can identify 91% of pregnancies that will develop preterm preeclampsia months ahead of symptoms, in women aged 35+ who do not have pre-existing high-risk conditions.
- Those with a low-risk result have 99.7% probability of not developing preterm preeclampsia.
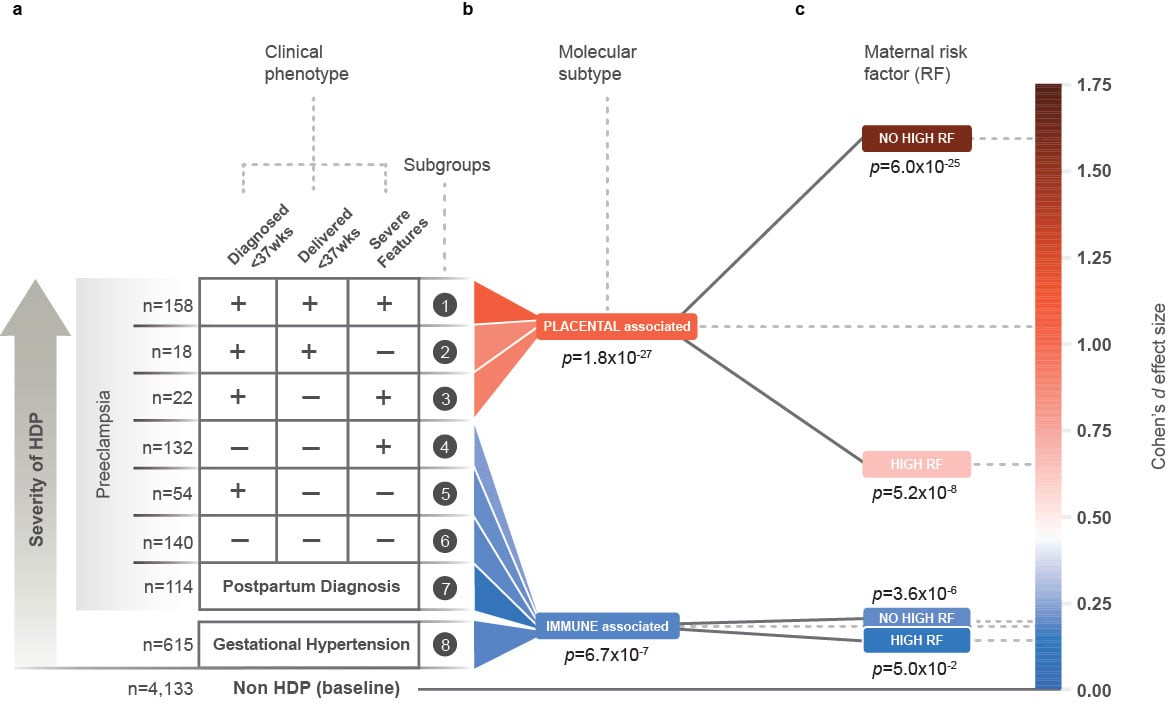
The study revealed that preeclampsia is associated with at least two distinct molecular subtypes. The results demonstrate the condition stems from diverse underlying molecular pathways, converging into a similar presentation. Preeclampsia has historically been considered a single condition, defined by its symptoms and clinical presentation. This allows, for the first time, a personalized medical approach to pregnancy and prenatal care.
.jpg)
Clinical validation of the placental molecular subtype demonstrates high performance, achieving 91% sensitivity with an AUC of 0.88 in predicting preterm preeclampsia in advanced maternal-age pregnancies.
Clinical significance
- Largest molecular study of its kind in pregnancy advances the biological understanding of hypertensive disorders of pregnancy, accelerating the potential for personalized care in obstetrics:
- Despite the deployment of current guidelines from U.S. Preventive Services Taskforce and the American College of Obstetrics and Gynecology that rely on maternal clinical and demographic characteristics to identify pregnant women as at increased risk for preeclampsia, rates of the disease have nearly doubled in the last decade and now affect 1 in 12 pregnancies.
- Mirvie’s test uses circulating RNA signatures to resolve this ambiguity, helping pregnant patients and their providers focus on the 1 in 4 pregnancies that are at high risk, ensuring optimal care for the right patients.
- Given that the adherence to known prophylactic interventions for pregnancies at high-risk of preeclampsia such as daily aspirin is less than 50%, even among high-risk patients, understanding risk at an individual level will promote and reinforce compliance with prophylaxis.
“Current guidelines are not helping us identify which patients are truly at high risk and we need better tools. Mirvie’s preeclampsia risk prediction test can now improve risk assessment, helping women and their care teams be informed and take actions with the potential to delay onset or prevent the disease.”
— Dr. Kara Rood
A maternal-fetal medicine physician, one of the principal investigators of the study, and Clinical Associate Professor of Obstetrics and Gynecology at The Ohio State University Wexner Medical Center.
- Low-risk result
- High-risk result
Understanding low-risk results
Low-risk results indicate there is a decreased risk of developing preeclampsia in this pregnancy.
Still, there are many things your patient can do to lower their risk for preeclampsia even more.
Each test report includes a customized plan that will provide options for you and them to do everything possible to prevent preeclampsia and recognize it right away if it does occur.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Reported health history
Each test report provides a summary of the individual's health history, focusing on major risk factors for preeclampsia.
It is essential to interpret test results while considering other clinical factors and evaluation for additional pregnancy complications should align with guideline recommendations.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Customized care plans
Each test report features a personalized care plan and recommendations tailored to the patient's test results, including a summary and a list of in-depth actions for each evidence-based prevention strategy.
These recommendations can significantly differ depending on whether the result indicates low or high risk.
Explore these evidence-based strategies further in:
"Care Plan for Individuals at Risk of Preeclampsia: a Collaborative Approach to Education, Prevention Strategies, Surveillance, and Follow-Up" by J. Roberts et al. (AJOG 2023).
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Risks and warning signs
Whether your patient is at low or high risk, it is crucial that they comprehend the potential dangers of preeclampsia.
Both you and the patient need to stay alert for any signs and symptoms and promptly identify them if they do arise. This proactive approach enables swift action for the best possible care for both the mother and the baby.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
More information
In a clinical validation study, Encompass identified 9 out of 10 eligible pregnancies that developed preeclampsia between 20 and 37 weeks, months in advance.
The performance characteristics of the Encompass test have been established in pregnant individuals age 35 or older and without a pre-existing major risk factor for preeclampsia, with a singleton pregnancy between 17 weeks 4 days and 22 weeks 0 days gestation.
Given this test is intended for screening preeclampsia risk, a "high-risk" result does not guarantee an affected pregnancy, and a "low-risk" result does not guarantee an unaffected pregnancy.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Patient name: Jane Doe
Encompass result:
Low Risk
There is a decreased risk of developing
preeclampsia in this pregnancy.
With a "low-risk" result, Encompass Care is recommended for your pregnancy. Although your risk of preeclampsia is low, there are still steps you can take to help have a healthy pregnancy. Discuss your recommended care plan with your provider.
Patient name: Jane Doe
Medical history and risks
Jane, you also reported no major risk factors for preeclampsia in your history. This is reassuring, but there are still steps you can take to lower your risk for preeclampsia even more in your recommended care plan.
Patient name: Jane Doe
Your suggested care plan
Review the specific recommendations outlined in your "low-risk" care plan with your provider to determine the best plan for your pregnancy. This is a brief summary:
- Medications: Based on your results, you don't require any medications. However, some providers may still suggest you take baby aspirin.
- Monitoring and labs: It's important to know the signs and symptoms of preeclampsia and get your blood pressure checked regularly.
- Nutrition: The Mediterranean diet can lower preeclampsia risk. Vitamin D and calcium are also important.
- Exercise: Exercise can lower your preeclampsia risk significantly.
- Sleep: While it may seem simple, it can make a difference.
Patient name: Jane Doe
Understanding your risk
Preeclampsia can cause serious complications for both moms and babies. Even with a "low-risk" result, a small percentage of people will still develop preeclampsia, and there are certain symptoms you should to be aware of.
When there are symptoms, they may include:
- Headache that will not go away
- Shortness of breath
- Pain in the abdomen or shoulder
- Nausea or vomiting in the second half of pregnancy
- Seeing spots, blurry vision, changes in eyesight
- Swelling of face or hands
- Sudden weight gain
- High blood pressure
Patient name: Jane Doe
About the test
Encompass is a screening test, not a diagnostic. It evaluates gene expression levels in the maternal blood, which is a mixture of maternal, fetal and placental origin, to predict the chance for preeclampsia.
The test isolates RNA from the maternal blood, from which libraries are created, sequenced and analyzed using Mirvie’s proprietary bioinformatics pipeline.
Note: This is a mock test report. Clinical performance data is available upon request.
Understanding high-risk results
High-risk results indicate there is an increased risk of developing preeclampsia in this pregnancy.
It is critical you and your patient work together to implement a care plan based on evidence-based strategies for preventing preeclampsia.
Each test report includes a customized plan that will provide options for you and them to do everything possible to prevent preeclampsia and recognize it right away if it does occur.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Reported health history
Each test report provides a summary of the individual's health history, focusing on major risk factors for preeclampsia.
It is essential to interpret test results while considering other clinical factors and evaluation for additional pregnancy complications should align with guideline recommendations.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Customized care plans
Each test report features a personalized care plan and recommendations tailored to the patient's test results, including a summary and a list of in-depth actions for each evidence-based prevention strategy.
These recommendations can significantly differ depending on whether the result indicates low or high risk.
Explore these evidence-based strategies further in:
"Care Plan for Individuals at Risk of Preeclampsia: a Collaborative Approach to Education, Prevention Strategies, Surveillance, and Follow-Up" by J. Roberts et al. (AJOG 2023).
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Risks and warning signs
Whether your patient is at low or high risk, it is crucial that they comprehend the potential dangers of preeclampsia.
Both you and the patient need to stay alert for any signs and symptoms and promptly identify them if they do arise. This proactive approach enables swift action for the best possible care for both the mother and the baby.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
More information
In a clinical validation study, Encompass identified 9 out of 10 eligible pregnancies that developed preeclampsia between 20 and 37 weeks, months in advance.
The performance characteristics of the Encompass test have been established in pregnant individuals age 35 or older and without a pre-existing major risk factor for preeclampsia, with a singleton pregnancy between 17 weeks 4 days and 22 weeks 0 days gestation.
Given this test is intended for screening preeclampsia risk, a "high-risk" result does not guarantee an affected pregnancy, and a "low-risk" result does not guarantee an unaffected pregnancy.
This is a mock test report. Your patient will receive one of two possible test results, either “low risk” or “high risk”. Explore each report by clicking the tabs.
Patient name: Jane Doe
Encompass result:
High Risk
There is an increased risk of developing preeclampsia in this pregnancy.
With a "high risk" result, Encompass Care Plus is recommended for your pregnancy. Remember, this result does not mean you will develop preeclampsia. In fact, with your recommended plan, you'll have tools to try to prevent it. Make sure to review it with your provider.
Patient name: Jane Doe
Medical history and risks
Jane, you reported no major risk factors for preeclampsia, but it's important to note that even individuals without major risk factors can still develop it. This is where your Encompass 'high-risk" result is helping you to know your objective risk for preeclampsia.
Patient name: Jane Doe
Your suggested care plan
Review the specific recommendations outlined in your "high-risk" care plan with your provider to determine the best plan for your pregnancy. This is a brief summary:
- Medications: Aspirin has been shown to be a safe and effective medication to lower your preeclampsia risk. Continue taking it if your doctor already recommended it or ask them if you should start it if you aren't.
- Monitoring and labs: High blood pressure is silent, but it is often the first sign of preeclampsia, so you must check it regularly. Your provider may also want to check your liver and kidney function and for protein in the urine.
- Nutrition: Healthy nutrition can have a big impact on your pregnancy and preeclampsia risk. The Mediterranean diet can lower preeclampsia risk. Vitamin D and calcium are also important.
- Exercise: Exercise can significantly lower your preeclampsia risk. It's best to combine aerobic and strength training.
- Sleep: Sleep is an important part of prevention. While it may seem simple, it can make a difference.
Patient name: Jane Doe
Understanding your risk
Preeclampsia can harm both moms and babies. For moms, it can lead to seizures, stroke, kidney and liver damage, and an increased risk of excessive blood loss during delivery. For babies, it can cause them to be smaller than normal and born prematurely. This might lead to a NICU stay or a longer stay in the hospital.
Preeclampsia can sometimes be silent, but there are certain symptoms to be aware of. Let your healthcare provider know if you experience any of the symptoms below. If your blood pressure is 140/90 or higher (for either number), contact your provider. If your blood pressure is 160/110 or higher (for either number), it could be an emergency, and you need immediate medical attention.
When there are symptoms, they may include:
- Headache that will not go away
- Shortness of breath
- Pain in the abdomen or shoulder
- Nausea or vomiting in the second half of pregnancy
- Seeing spots, blurry vision, changes in eyesight
- Swelling of face or hands
- Sudden weight gain
- High blood pressure
Patient name: Jane Doe
About the test
Encompass is a screening test, not a diagnostic. It evaluates gene expression levels in the maternal blood, which is a mixture of maternal, fetal and placental origin, to predict the chance for preeclampsia.
The test isolates RNA from the maternal blood, from which libraries are created, sequenced and analyzed using Mirvie’s proprietary bioinformatics pipeline.
Note: This is a mock test report. Clinical performance data is available upon request.
About the test
The Encompass test is performed by a CLIA-certified lab to screen for preeclampsia risk.
Patients should always discuss their results and action plans with their care team.
Encompass is a screening test, not a diagnostic. It evaluates gene expression levels in the maternal blood, which is a mixture of maternal, fetal and placental origin, to predict the chance for preeclampsia.
The test isolates RNA from the maternal blood, from which libraries are created, sequenced and analyzed using Mirvie’s proprietary bioinformatics pipeline.
The performance characteristics of the Encompass test have been established in pregnant individuals age 35 or older and without a pre-existing major risk factor for preeclampsia, with a singleton pregnancy between 17 weeks 4 days and 22 weeks 0 days gestation.
References:
Rasmussen M, et al. RNA profiles reveal signatures of future health and disease in pregnancy. Nature, 601:422-427 (2022)
Roberts J, et al. Care plan for individuals at risk for preeclampsia: shared approach to education, strategies for prevention, surveillance, and follow-up. Am J Obstet Gyn, 229(3):193-213 (2023)
Elovitz et al. Molecular Subtyping of Hypertensive Disorders of Pregnancy. Nature Communications (2025)16:2948
Connect with
our team.

 Carrie Haverty, MS, CGC
Carrie Haverty, MS, CGC
